ican711nm wrote:parados wrote:
...
The net effect HAS been constant for centuries until humans started adding CO2.

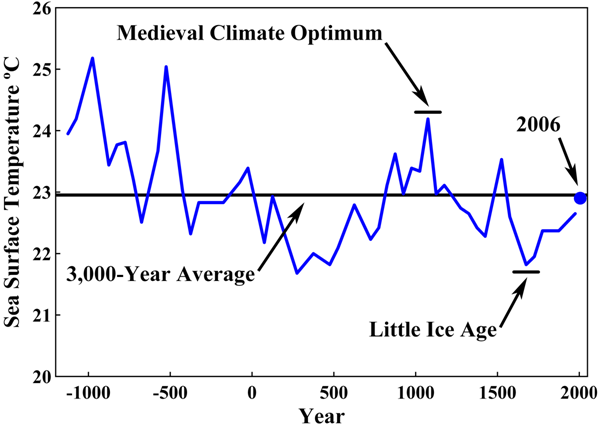
A 3000+ year look at Sargossa sea level temperatures up to 2006
Hey, ican..
If you don't know the difference between CO2 levels and temperature what the hell are you doing here?
Other than making an ass of yourself?
The CO2 levels were relatively constant for centuries. If you bothered to include my entire quote it would have been obvious what I was talking about.
parados wrote:ican711nm wrote:parados wrote:
...
The net effect HAS been constant for centuries until humans started adding CO2.


A 3000+ year look at Sargossa sea level temperatures up to 2006
...
The CO2 levels were relatively constant for centuries. If you bothered to include my entire quote it would have been obvious what I was talking about.
parados wrote:The net effect HAS been constant for centuries until humans started adding CO2. I am not the one making the mistake okie. It is you. Human CO2 doesn't cause the release of more natural CO2 or at least I see no evidence of that. What evidence do you have that human caused CO2 causes more natural CO2? Until you provide any evidence "net effect" and occam's razor applies as the prevailing logic.
CO2 ppm in the atmosphere has not been relatively constant for centuries before humans started releasing CO2.
Quote:
http://en.wikipedia.org/wiki/Carbon_dioxide#In_the_oceans
In the oceans
There is about 50 times as much carbon dissolved in the oceans in the form of CO2 and CO2 hydration products as exists in the atmosphere. The oceans act as an enormous carbon sink, having "absorbed about one-third of all human-generated CO2 emissions to date[23]." Generally, gas solubility decreases as water temperature increases. Accordingly carbon dioxide is released from ocean water into the atmosphere as ocean temperatures rise.
More CO2 is released from ocean water as ocean temperatures rise and more CO2 goes into ocean water as ocean temperatures fall. The rising and falling of the temperature of the Sargossa Sea is only part of the evidence that ocean temperatures have not been relatively constant for centuries. Therefore, since the temperatures of ocean waters have not been relatively constant for centuries, the amount of CO2 in the atmosphere has not been relatively constant for centuries.
It is possible to measure the amount of CO2 in the atmosphere, on a scale of resolution of decades, directly from the small trapped bubbles of atmosphere in ice cores. Those samples are the actual atmosphere, ican, they are NOT CO2 in water, as you seem to think, and CO2 is not the only component of the atmosphere that has been measured--it is merely the one we're concerned with here. And what the those cores show is that during ice ages CO2 is relatively constant at around 180 ppm, and during interglacials, as today, CO2 has been relatively constant in each, with values ranging from about 260ppm in some interglacials, to about 300ppm in one of them, but in most of them around 280.
Parados is right. You are not. This interglacial was pretty much 280ppm for the last several thousand years. Nowehere in the record for the last 800,000 years has there been a CO2 level approaching today. And what one thing producing CO2 is different in the last century and a half of this interglacial than in the previous ten thousand years of it and in the last seven interglacials?
Citing sea surface temperatures of the Sargasso Sea proves nothing. The Sargasso Sea is an anomaly. It is formed by a loop of the Gulfstream running beneath it. The Gulfstream is warmer than the surrounding ocean. It runs by different conditions than the surrounding ocean. Things can happen to it that aren't representative of the earth as a whole. Try googling "thermohaline circulation" at Wikipedia for an example of the kinds of thing that can make the Gulfstream and its northern extension the NAD non-representative of what's going on in the world as a whole.
I invite you once again to do the math on outgasing with respect to increasing temperature. You haven't, and consequently you keep repeating the canard that the ocean is outgassing enough CO2 to make up the change in the atmosphere. If you look at a graph of decreasing solubility with increasing temperature for CO2 (they're different for different gases), you'll find that a 1 degree C increase in temp (which is what we've had in the last century) would produce about a 2% drop in solubility OVER THE COURSE OF THAT CENTURY, NOT EACH YEAR). However over the same century the capacity of oceans to absorb CO2 has increased by about a third, which under gas laws is proportional to the increase of CO2 in the atmosphere. You seem to be unfamiliar with the concept of a carbon sink. A sink absorbs something. If the net effect is that it releases more than it absorbs, it is not a sink. The oceans have been, and continue to be carbon sinks. According to NOAA, they aborb 92.4 Gt C every year, and release 90.8 back to the atmosphere, year after year after year. That, my boy, is what a sink does. Oceans would only have released CO2 because of an increase in temperature if they were at saturation, because that temp change reduces the total capacity of a liquid to hold a gas. The amount in a saturated solution that is above the solubility level of the increased tempreature gets released. However theo oceans aren't at saturation, or they wouldn't keep absorbing CO2.. They do, and their capacity keeps increasing because CO2 in the atmosphere keeps going up. Since you love simplistic linear relationships so much, 92.4/90.8 produces a net gain of CO2 by the oceans of about 1.6% a year, YEAR AFTER YEAR, versus your ONE-TIME-ONLY over-the-couse-of-a-century total 2% outgasing for temp. rise. So claiming the increase in CO2 comes from the oceans is just plain nonsensTSI can't account for it either. As noted above, the sun's output has decreased since 1975, while earth tempreature has risen. A decrease in energy input is not going to account for an increase in energy.
Sorry, bucko, the bird still don't fly.
I also found it ironic that miniTax, in an effort to prove his point, last introduced a grph that certainly seems to prove mine. His graph showed quite clearly that the amount of CO2 the oceans have been absorbing has increased over the last century, as we would expect with the increase in CO2 in the atmosphere increasing its partial pressure, increasing the capacity of the oceans to hold more CO2. Thank you, miniTAX, for proving my point, inadvertently I'm sure.
ican711nm wrote:CO2 ppm in the atmosphere has not been relatively constant for centuries before humans started releasing CO2.
Quote:
http://en.wikipedia.org/wiki/Carbon_dioxide#In_the_oceans
In the oceans
There is about 50 times as much carbon dissolved in the oceans in the form of CO2 and CO2 hydration products as exists in the atmosphere. The oceans act as an enormous carbon sink, having "
absorbed about one-third of all human-generated CO2 emissions to date[23]." Generally, gas solubility decreases as water temperature increases. Accordingly carbon dioxide is released from ocean water into the atmosphere as ocean temperatures rise.
Damn ican. That quote directly contradicts your claims about the human produced CO2. If the oceans only absorbed 1/3 of all human generated CO2 emissions to date then that means 2/3 are someplace OTHER than the oceans. Perhaps the atmosphere?
Quote:
More CO2 is released from ocean water as ocean temperatures rise and more CO2 goes into ocean water as ocean temperatures fall. The rising and falling of the temperature of the Sargossa Sea is only part of the evidence that ocean temperatures have not been relatively constant for centuries. Therefore, since the temperatures of ocean waters have not been relatively constant for centuries, the amount of CO2 in the atmosphere has not been relatively constant for centuries.
I guess I shouldn't worry about you making an ass of yourself if you aren't worried about it.
parados wrote:ican711nm wrote:CO2 ppm in the atmosphere has not been relatively constant for centuries before humans started releasing CO2.
Quote:
http://en.wikipedia.org/wiki/Carbon_dioxide#In_the_oceans
In the oceans
There is about 50 times as much carbon dissolved in the oceans in the form of CO2 and CO2 hydration products as exists in the atmosphere. The oceans act as an enormous carbon sink, having "
absorbed about one-third of all human-generated CO2 emissions to date[23]." Generally, gas solubility decreases as water temperature increases. Accordingly carbon dioxide is released from ocean water into the atmosphere as ocean temperatures rise.
Damn ican. That quote directly contradicts your claims about the human produced CO2. If the oceans only absorbed 1/3 of all human generated CO2 emissions to date then that means 2/3 are someplace OTHER than the oceans. Perhaps the atmosphere?
There is probably some in the atmosphere, but I imagine most of the rest has been absorbed by plants, into the soil etc. To presume that any not absorbed into the ocean is loose in the atmosphere is rather tunnel visioned I think.
Foxfyre wrote:
There is probably some in the atmosphere, but I imagine most of the rest has been absorbed by plants, into the soil etc. To presume that any not absorbed into the ocean is loose in the atmosphere is rather tunnel visioned I think.
No, it would be science and math.
To presume that the "soil" absorbs CO2 in a quantity equal to 2/3 of what humans release would be looney.
Plants have a cycle. They take up CO2 then die, decay and give off the CO2. Until you can come up with some way for plants to take up CO2 and not give it off in about the same quantity they take it up your idea in that area doesn't hold much CO2.
The net effect of vegetation is they may sequester some CO2 but for the most part they give off as much as they take up.
username wrote:It is possible to measure the amount of CO2 in the atmosphere, on a scale of resolution of decades, directly from the small trapped bubbles of atmosphere in ice cores.
Username, that's false. You'll find no CO2 ice records older than thousands years ago with decades resolution. NONE. If you do, you should show your data, otherwise, it's just a nonsensical claim.
If you can't even get such simple facts right, how can you get more complex things right like reactive decay, isotope dosage, diffusion filtering effects... ?
I don't want to be rude but it's tiring to keep rectifying AGW nonsense.
username wrote:I also found it ironic that miniTax, in an effort to prove his point, last introduced a grph that certainly seems to prove mine. His graph showed quite clearly that the amount of CO2 the oceans have been absorbing has increased over the last century, as we would expect with the increase in CO2 in the atmosphere increasing its partial pressure, increasing the capacity of the oceans to hold more CO2. Thank you, miniTAX, for proving my point, inadvertently I'm sure.
Your point is to simplify and exagerate a complex and ill understood scientific problem (the CO2 cycle) to contend the debate is "over".
I'm not sure you should thank me for that

miniTAX wrote: Username, that's false. You'll find no CO2 ice records older than thousands years ago with decades resolution. NONE. If you do, you should show your data, otherwise, it's just a nonsensical claim.
What is your opinion of historical CO2 measurements, in terms of just how far back can we safely go with it?
Parados has claimed that atmospheric CO2 has been relatively constant for centuries. We know that there is evidence in geologic history that CO2 was possibly several thousand ppm in the atmosphere, many times what it is now, so the implication that CO2 should or would have stayed constant without the influence of man is a fallacy.
parados wrote:Foxfyre wrote:
There is probably some in the atmosphere, but I imagine most of the rest has been absorbed by plants, into the soil etc. To presume that any not absorbed into the ocean is loose in the atmosphere is rather tunnel visioned I think.
No, it would be science and math.
To presume that the "soil" absorbs CO2 in a quantity equal to 2/3 of what humans release would be looney.
Plants have a cycle. They take up CO2 then die, decay and give off the CO2. Until you can come up with some way for plants to take up CO2 and not give it off in about the same quantity they take it up your idea in that area doesn't hold much CO2.
The net effect of vegetation is they may sequester some CO2 but for the most part they give off as much as they take up.
So you're saying plants emit as much CO2 as they take in? And you studied biology where? We were also taught that more carbon is retained in the soil than in all the plants and oceans combined. I also didn't say or presume that the soil absorbs CO2 in a quantity equal to 2/3 of what humans release.
You however seem to assume that the 2/3rds is still suspended in the atmosphere which is a far greater assumption than assuming that much if not most of it is contained in plants and soil.
Foxfyre wrote:
So you're saying plants emit as much CO2 as they take in? And you studied biology where?
Actually - especially during heatwaves - plants even produce more carbon dioxide than they absorbed from the atmosphere. (see Nature, 19 Sep 2005).
And you studied biology where, Foxfyre, you said?
(I just had biology in school, though up to the final exams [Abitur].)
I studied biology before September 2005. But that is interesting. I wonder then why the environmental gurus at Kyota et al are offering countries with large amounts of vegetation carbon credits because they are removing so much of the CO2 they are producing? Hmmmm?
Foxfyre wrote:I studied biology before September 2005.
Well, I left school in 1969 - but that didn't prevend me from reading :wink:
okie.
A century is 100 years.
Thousands of years would be well over 10 centuries.
Geological history of high CO2 would be well past the thousands of years mark.
Walter Hinteler wrote:Foxfyre wrote:I studied biology before September 2005.
Well, I left school in 1969 - but that didn't prevend me from reading :wink:
Perhaps leaving school in 1969 explains why you pick one study suggesting it is proof (when most textbooks and other sources state otherwise) and also why you avoided the question re those carbon credits that was rather pertinent to this discussion. You see, I read those textbooks and I read just about every source that crosses my path re this subject.
Foxfyre wrote:parados wrote:Foxfyre wrote:
There is probably some in the atmosphere, but I imagine most of the rest has been absorbed by plants, into the soil etc. To presume that any not absorbed into the ocean is loose in the atmosphere is rather tunnel visioned I think.
No, it would be science and math.
To presume that the "soil" absorbs CO2 in a quantity equal to 2/3 of what humans release would be looney.
Plants have a cycle. They take up CO2 then die, decay and give off the CO2. Until you can come up with some way for plants to take up CO2 and not give it off in about the same quantity they take it up your idea in that area doesn't hold much CO2.
The net effect of vegetation is they may sequester some CO2 but for the most part they give off as much as they take up.
So you're saying plants emit as much CO2 as they take in?
Yes, I am saying that. Do you know what decay is? Decay causes the carbon trapped in vegetable matter to be released as CO2. It may take a year or 2 or even a 10 but eventually unless the vegetation is trapped in some way to prevent decay it will be released.
Quote:And you studied biology where? We were also taught that more carbon is retained in the soil than in all the plants and oceans combined.
Soil is made up of vegetative matter often in some form of decay.
If you were taught that the soil contains more carbon than the oceans and plants combined, I would ask for my money back.
http://www.geotimes.org/jan02/feature_carbon.html
Soils contain more carbon than plants and the atmosphere combined but the ocean contains 50 times the carbon of the atmosphere.
Also this
Quote: Carbon in soils under natural ecosystems often is at high levels and is considered at equilibrium, thus unable to sequester additional carbon.
Quote: I also didn't say or presume that the soil absorbs CO2 in a quantity equal to 2/3 of what humans release.
You did say this..
Quote:most of the rest has been absorbed by plants, into the soil etc.
It looks like you were claiming it was absorbed into the soil and plants. Since plants are a zero sum game with CO2 that would put soil as the main absorber in your statement.
Quote:
You however seem to assume that the 2/3rds is still suspended in the atmosphere which is a far greater assumption than assuming that much if not most of it is contained in plants and soil.
Considering the measurements of CO2 concentrations show that the atmosphere accumulates about 1/2 of the human emissions each year, I wouldn't call it an assumption.
okie wrote:What is your opinion of historical CO2 measurements, in terms of just how far back can we safely go with it?
With icecores, you get one point of measurement for CO2 or temperature every century or millenium thousands years ago. So suppose if there were a 30% increase in the distant past similar to the past 50 years, nobody would see it since it is filtered out : CO2 is supposed to be sealed in ice's bubbles but it is a pure speculation, CO2, like any other gases migrates, is disolved by water, reacts with dusts or other aerosols...
Worse, CO2 in icecores is supposed to reflect direct measurements which exist only since the 50's. But it is not! People who speculate it SUPPOSE that there is a 30 to 50 (or 100, 200...) year lag beetwen the icecore age and CO2 age but this lag is just a fudge factor to mix apples (CO2 by icecores) to orange (CO2 by direct modern instruments) : see columns 4 and 5 of the Law Dome icecore data for example :
http://cdiac.ornl.gov/ftp/trends/co2/lawdome.combined.dat .
And the AGW people use that crap to say the CO2 level is "unprecedented" whereas in fact, all that can be said is CO2 is unprecedented... since the 1950's. But hey, that's climate science !