@littlek,
Factors That Affect the Boiling Point
Pressure: when the external pressure is:
less than one atmosphere, the boiling point of the liquid is lower than its normal boiling point.
equal to one atmosphere, the boiling point of a liquid is called the normal boiling point.
greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point.
The following graph shows the boiling point for water as a function of the external pressure. The line on the graph shows the normal boiling point for water.
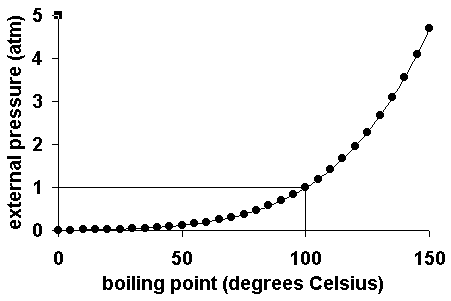
Closed lid = greater pressure inside pot then outside. Water boils faster.
No lid = pressure outside is = to pressure inside pot. Water boils at 100 degrees Celsius.